The vaccine transporters
The development of the first mRNA vaccines was a milestone in the struggle against the coronavirus pandemic. Evonik supplies a crucial technology for transporting these gene-based vaccines to the right target site in the human body: lipid nanoparticles. Without them, the vaccine would be ineffective.
Guiding the release of sensitive active ingredients inside the human body has been a specialty of Evonik for decades. As a specialty chemicals company, Evonik has been a global leader in the field of drug delivery technologies. It’s also participating in the struggle against the coronavirus, in close cooperation with vaccine producers such as BioNTech.
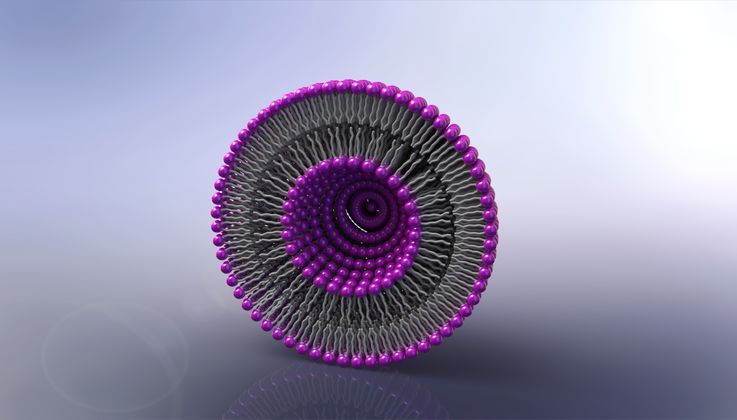
In Canada and the USA, Evonik has been developing and producing lipid nanoparticles (LNPs) for years. LNPs are absolutely essential for the effectiveness of mRNA-based vaccines: These ultrafine particles made of fats and waxes envelop the mRNA and transport it into human cells. Without the protective sheath of LNPs, the mRNA would disintegrate inside the body within seconds.
In order to be able to supply vaccine producers directly from a European location, Evonik is building its own lipid production facility in Hanau and Dossenheim. “In this way we are emphasizing our leading position as a development and contract partner of the global pharmaceutical industry,” says Thomas Riermeier, senior vice president & general manager of the Health Care business line.
The development of mRNA technology proceeded for many years largely unnoticed by the general public. Nonetheless, Evonik recognized the potential of gene-based therapeutic approaches early on. The company already develops and formulates lipid nanoparticles in close cooperation with pharmaceutical companies in the town of Burnaby near Vancouver (Canada). It also operates a plant for the production and bottling of commercial volumes of LNPs in Birmingham, Alabama (USA). In 2016 Evonik acquired the Canadian company Transferra Nanosciences, and at the beginning of 2020 it continued the expansion of its portfolio by acquiring Wilshire Technologies, an American producer of plant-based auxiliary materials for the pharmaceutical industry.
“Vancouver is an epicenter for the develop - ment and production of LNPs,” says Stefan Randl, who heads research at Evonik’s Health Care business line. Research on lipid nanoparticles has been conducted there for almost 30 years. “The researchers have already developed hundreds of LNP formulations for gene-based and cell-based therapies, and they are connected with pharmaceutical and biotech companies all over the world,” says Randl. To date, Evonik has provided support for a whole series of drugs that have been authorized or are currently in the development stage. Today LNP technology is regarded as the “gold standard” for the development of complex parenteral medications-in other words, those that are administered via injection - against diseases such as cancer. Certain combinations of active ingredients, as well as personalized medications, would be unthinkable without LNPs.
The covid-19 pandemic has greatly accelerated the development of innovative therapeutic approaches. In addition to the vaccines against covid-19, Evonik’s researchers are working in many other fields of application for mRNA that the company believes are close to a breakthrough. Innovative vaccines against the flu, malaria, and HIV are just as conceivable as promising approaches for cancer immunotherapy, the treatment of genetic diseases, and personalized medicine.